Status of the Stryker LFIT V40 Litigation
Ashleigh Raso of NGRV is on the Plaintiff’s Steering Committee for the Stryker LFIT V40 litigation. In 2017, Stryker LFIT V40 cases were consolidated into a Multidistrict Litigation (“MDL”) in federal court in Massachusetts. State court litigation also proceeded in New Jersey. When MDL’s are formed the Judge picks several counsel to lead the litigation. Ashleigh Raso was chosen to be Administrative Counsel in the Stryker LFIT V40 litigation.
In November of 2018, an order “Aiding Private Settlement” was filed in both the MDL and the New Jersey state court. A stay, which is an order halting the discovery process, was entered while the private settlement was processed. In December of 2021, another order “Aiding Private Settlement” was also entered. Currently there is a stay in discovery as to Defendant, but Plaintiffs must produce some materials and abide by certain court orders to file in the MDL.
Stryker V40 Hip Recall
In 2016, Stryker voluntarily recalled certain lots of the LFIT V40 Cobalt Chromium (CoCr) femoral heads. The femoral head is part of a hip replacement system and may be paired with a variety of stems. Stryker explained in its recall letter that the reason for recall was a high incidence of “taper lock failure”. At that time there was no recommended action by patients. However, in May of 2018, Stryker expanded its prior recall to include all LFIT V40 CoCr heads manufactured before March 3, 2011, size 36mm and above. In the letter Stryker explained that there had been a “higher than expected number of complaints documenting femoral head/hip stem dissociation” for certain sizes of the LFIT V40 Head.
Defect in the Stryker LFIT V40 Head:
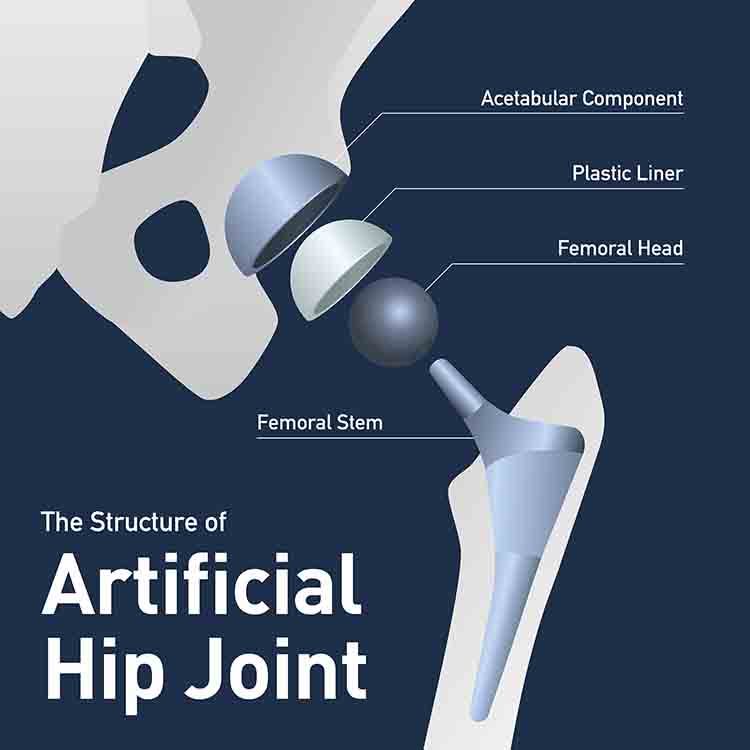
The LFIT V40 head, made from cobalt and chromium, is paired with the titanium femoral stem. The two parts are cold-welded together during hip replacement surgery.
Unfortunately, despite best efforts, it is nearly impossible to perfectly match the trunnion of the femoral stem to the femoral head. This results in micromotion between the two parts. Micromotion between metal parts can cause fretting of those metals, which can travel to the surrounding tissue and cause necrotic tissue, pseudotumor (a benign tumor-like appearance), or in severe cases, head dissociation. In the cases of head dissociation, the trunnion is worn down to a “pencil tip” or “bird beak” appearance.

Stryker LFIT V40 Injuries
In severe cases, those who have received an LFIT V40 head will need to undergo revision surgery. Your doctor may do blood tests first to measure how much cobalt and chromium has reached your blood stream. Your doctor may also order an MRI to detect any pseudotumors that may be present. It is important that you call a lawyer before your revision surgery so that the explant- the device taken out during surgery- is preserved.
Stryker V40 Lawsuit
If you or a loved one have been implanted with a Stryker LFIT V40 head and have or need to undergo revision surgery, call our Stryker V40 hip attorneys at 202-792-7927 today.