Covidien Hernia Mesh Lawsuits
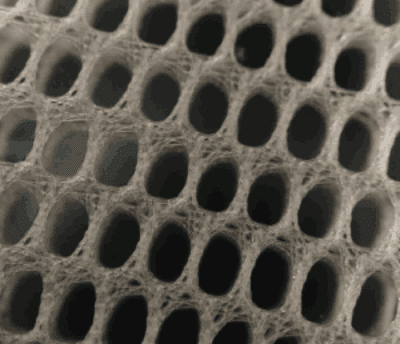
Covidien is the second largest hernia mesh manufacturer in the world after Bard. In 2014, Covidien was acquired by Medtronic for $42.9 billion. Covidien is located in Massachusetts and Medtronic is located in Minnesota. Like most hernia mesh manufacturers in the United States, Covidien sells polypropylene-based hernia meshes. However, Covidien is the only hernia mesh manufacturer in the United States to sell polyester-based hernia meshes. Covidien primarily sells its polypropylene hernia mesh devices overseas and primarily sells its polyester-based hernia mesh in the United States.
Covidien Hernia Mesh Lawsuit
Covidien and Medtronic are facing several thousand lawsuits regarding their polypropylene and polyester hernia mesh devices in several jurisdictions. There are hernia mesh lawsuits pending against Covidien and Medtronic in Minnesota state court, Massachusetts state court, and Massachusetts federal court.
Covidien Massachusetts State Court Lawsuits
Lawsuits against Covidien for their hernia mesh were initially filed in Massachusetts state court where Covidien is headquartered. Over 6,000 hernia mesh claims are now filed against Covidien just in Massachusetts state court. The Covidien hernia mesh claims in Massachusetts state court are split up into two main buckets:
- Multifilament polyester hernia meshes
- Monofilament polyester hernia meshes
Covidien’s polypropylene hernia meshes are not subject to litigation in Massachusetts state court. Many consider polyester to be an inferior alternative to polypropylene. Polyester-based hernia meshes degrade as much as or more than polypropylene hernia meshes after implanted in the human body. Polyester hernia meshes are also incredibly susceptible to becoming infected. Multifilament polyester hernia meshes increase the rate of chronic infections even more, luckily most polyester hernia meshes are no longer being sold or implanted. Massachusetts state court was initially only focused on multifilament polyester hernia mesh lawsuits.
Covidien Minnesota State Court Lawsuits
- Bare polypropylene or polyester hernia meshes
- Polypropylene or polyester hernia meshes with a resorbable adhesion barrier
- Polypropylene or polyester hernia meshes with self-fixating microgrips

Unlike the Massachusetts state court action, the Minnesota state court action includes polypropylene-based hernia meshes and does not focus on if the polyester is monofilament or multifilament. The Covidien hernia mesh state court litigation is currently in the discovery phase. The defendants are producing large volumes of documents related to their hernia meshes every week.
Covidien Multi-District Litigation (MDL) 3029
The Covidien hernia mesh MDL is located in Massachusetts federal court and is known as MDL 3029 (In re Covidien Hernia Mesh Prods. Liab. Litig. No. II; MDL 3029 (D. MA). The Honorable Patti B. Saris serves as a United States District Judge for the District of Massachusetts and is leading the Covidien hernia mesh MDL. As of December 19, 2023, there are approximately 830 Covidien hernia mesh lawsuits on file in the Covidien MDL 3029. The Honorable M. Page Kelley serves as the Chief Magistrate Judge for the Covidien hernia mesh MDL. Judge Kelly appointed leadership for the Covidien hernia mesh MDL via case management order 10. Brett Vaughn from Nigh Goldenberg Raso & Vaughn was court appointed to the Plaintiffs’ Executive Committee (PEC). The Covidien hernia mesh MDL has already decided the following hernia mesh types will be the first trials (bellwether trials):
- Progrip
- Symbotex
Both the Covidien Progrip and Symbotex hernia meshes are monofilament polyester designs that are still sold and implanted in humans. The Symbotex is a hernia mesh with a resorbable adhesion barrier that is intended to protect the bowels and other organs from the dangerous underlying polyester mesh. The Symbotex is typically used for ventral/abdominal wall hernias. The ProGrip is a hernia mesh with thousands of microgrips intended to permanently attach the hernia mesh to underlying tissues. The ProGrip is typically used for inguinal hernias.
What Is Wrong With Covidien Hernia Mesh?

Covidien’s hernia mesh devices were cleared by the FDA, which is very different than being FDA-approved. FDA-approved devices must undergo pre-market human studies and are then approved by the FDA as being safe and effective in humans before the devices can be marketed. FDA-cleared devices just have to show that they are substantially equivalent to devices already on the market, and then the FDA clears the devices for sale. No pre-market human studies were conducted on Covidien hernia mesh products and they have not been approved by the FDA as safe and effective. Covidien hernia mesh can result in many post-operative complications. These are the most commonly reported complications associated with the use of Covidien hernia mesh:
-
- Chronic mesh infections lasting months to years. Infections are not cleared by oral antibiotics. Long courses of IV antibiotics are frequently required. Wounds related to the hernia mesh do not heal or they reopen. Many patients require wound-vac treatment in conjunction with their infection treatment.
- Excessive inflammation at the site of the mesh implant. Excessive inflammation causes undesired scar tissue and weaker collagen to form. This can result in adhesions and nerves being caught up in the scar tissue. Excessive inflammation can lead to chronic pain and increased rates of hernia recurrence.
- Chronic pain after the mesh has been implanted for more than 3 months. Many patients experience disabling pain after being implanted with Covidien hernia mesh products. Rates of disabling pain seem to be alarmingly high with the ProGrip mesh.
- Seroma or an excess of fluid that can lead to swelling, discomfort, and infection.
- Fistula is an abnormal connection or passageway between two organs or an organ and the surface of the skin. Fistulas are frequently associated with mesh infections. Mesh explantation is typically required if the mesh is involved in a fistula.
- Adhesions are dense scar tissues that attach the hernia mesh to organs, such as the bowel. Dense adhesions can cause chronic pain, change in bowel habits, and bowel obstructions. Many patients have required bowel resections as a result of dense and fibrotic hernia mesh adhesions.
- Mesh migration, erosion or rejection from the body.
If you or a loved one has experienced any of these hernia mesh complications after being implanted with a Covidien hernia mesh, do not hesitate to speak to our hernia mesh lawyers who will determine if you qualify for a Covidien hernia mesh lawsuit or settlement.
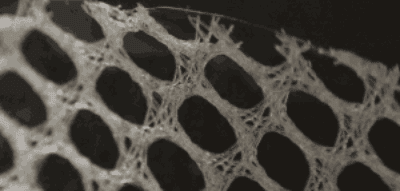
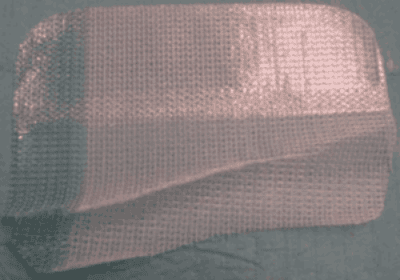
Which Covidien Hernia Mesh Products Have Been Linked to Complications?
- Parietex Hernia Mesh

The Parietex hernia mesh was previously made of multifilament polyester. The multifilament polyester version of Parietex had incredibly high rates of infection. Parietex monofilament polyester hernia mesh also tears easier and is prone to a higher recurrence rate. The Parietex hernia mesh is used for both ventral and inguinal hernias. Covidien continues to sell the monofilament Parietex hernia mesh.
- Parietex Composite Hernia Mesh (PCO)

The Parietex Composite hernia mesh has evolved overtime, including a Parietex Optimized Composite mesh (PCOx). The PCO line has included both monofilament and multifilament polyesters and is associated with high rates of infection. The PCO line also includes a resorbable adhesion barrier to prevent dense scar tissue from connecting the mesh to underlying organs. The Parietex Composite hernia mesh is typically used on ventral and umbilical hernias. Our hernia mesh attorneys allege that the adhesion barrier of the Parietex composite mesh does not last long enough and does not protect underlying organs from the dangerous polyester mesh. As a result, many patients experience bowel obstructions, infections, fistulas, and bowel resections. Covidien no longer sells the original Parietex Composite hernia mesh, but does continue to sell the Parietex Optimized Composite hernia mesh.
- ProGrip Hernia Mesh

The Parietex ProGrip hernia mesh utilizes monofilament polyester (there is also a Parietene ProGrip that utilizes polypropylene as a base material). The ProGrip hernia mesh is advertised as resulting in less chronic pain, because it doesn’t need to be sutured or tacked into place. Instead, the ProGrip is equipped with thousands of micro-grips that act like velcro, adhering the mesh to the patient’s tissues. These micro-grips cause profound inflammation as they resorb and make the mesh nearly impossible to remove if complications arise. Many patients experience debilitating pain and have to have many surgeries where they surgeon painstakingly “piece-meals” out the mesh, like pulling bubble gum out of hair. While advertised as a hernia mesh that would result in less pain, our hernia mesh attorneys allege that the ProGrip hernia mesh causes the highest rates of chronic debilitating pain. Covidien continues to sell the ProGrip hernia mesh.
- Symbotex Hernia Mesh

The Symbotex is a very light-weight monofilament polyester hernia mesh with a resorbable adhesion barrier coating. The Symbotex hernia mesh is prone to the mesh blowing out or tearing, resulting in recurrences and bowel injuries. The adhesion barrier of the Symbotex also frequently fails to prevent the formation of adhesions to the underlying polyester mesh. Revision surgeries can last for hours as the surgeon tediously attempts to remove the mesh from the patient’s bowel. Many patients require parts of their bowel removed along with the failed hernia mesh. Covidien continues to sell the Symbotex hernia mesh.
Timeline of the Covidien Hernia Mesh Lawsuits – Updates & History
June 2024
The various Covidien hernia mesh lawsuits continue on in the discovery phase of the litigation. To date, nearly 2 million documents have been produced regarding the Covidien hernia mesh products. Depositions have begun of corporate witnesses involved with Covidien hernia mesh. Because most of the meshes that Covidien sells in the United States were designed and developed in Europe, most of the depositions are taking place in Brussels. The discovery deadline in the Covidien MDL is currently set for the end of 2024, at which time expert reports will be due.
June 2023
On June 14, 2023, counsel for both the plaintiffs and the defense presented a Science Day to the Court. Plaintiffs’ presentation included an animated video on both the ProGrip hernia mesh and the Symbotex hernia mesh and the complications that can result.
April 2023
The parties in the MDL have selected which Covidien hernia mesh cases will go to trial first. All plaintiffs have been implanted with either a ProGrip or Symbotex hernia mesh.
December 2022
As of December 15, 2022, 156 cases are pending in federal courts against Covidien in relation to their hernia mesh products.
October 2022
A plaintiff’s submission on October 14, 2022, proposed for the discovery to be completed by April 8, 2024. On the contrary, Covidien proposed for the corporate discovery to be completed by September 30, 2023, giving the plaintiffs less than a year in total. The plaintiffs opposed the defendant’s proposal.
August 2022
The first status conference took place, just two months after the consolidated MDL (90 cases pending). The next steps include completing the corporate discovery and scheduling the first dates of the initial bellwether cases.
June 2022
This time the JPML decided to form the Covidien hernia mesh lawsuits into a mass action MDL against Covidien and Medtronic. The main reasons in favor of this decision were:
-
- The vast increase of plaintiffs alleging injury from Covidien hernia mesh (85 plaintiffs compared to 12 in 2020)
-
- The similarity of the Covidien hernia mesh cases / practicality reasons
-
- The support of the Covidien hernia mesh plaintiffs to create the MDL
-
- The projections of the panel for a significant increase in hernia mesh cases against Covidien being filed in federal court
-
- The significant increase in state court hernia mesh cases against Covidien, resulting in the need for federal-state coordination
The federal Covidien hernia mesh lawsuits were consolidated into a mass action MDL in the District of Massachusetts, where Covidien is headquartered and most cases were pending at the time. Judge Patti B. Sarris is appointed to preside over all federal Covidien hernia mesh lawsuits.
July 2020
The Judicial Panel for Multi-District Litigations (JPML) denied centralizing the Covidien hernia mesh lawsuits into a Multi-District Litigation (MDL).
After considering multiple factors, including the number of Covidien hernia mesh cases on file, the similarities of the factual and legal issues involved in the Covidien hernia mesh cases on file, and the convenience of the parties and witnesses, the judicial panel decided not to transfer the Covidien hernia mesh cases to a single federal district court for pretrial proceedings. The main reason for the rejection was the low number of Covidien hernia mesh plaintiffs on file in federal court (12 Covidien hernia mesh actions were pending in federal court at the time).
Covidien Hernia Mesh MDL 3029 Case Management Orders
All case management orders (CMOs) for the Covidien hernia mesh MDL 3029 can be found here. Case management orders are a great way to stay up to date on a litigation.
Am I Eligible to File a Lawsuit Against Covidien for Their Defective Hernia Mesh?
If you have experienced complications or injuries as a result of a defective hernia mesh product manufactured by Covidien, you may be eligible to file a lawsuit against the company.
Things your hernia mesh lawyer will have to show or consider when filing your hernia mesh lawsuit:
-
- Demonstrate that the hernia mesh product was defective and that the defect caused injury or harm. A lawyer can help you dig up your medical history and find out which specific hernia mesh product was used during the hernia repair.
-
- Show that your doctor used the product as intended or advertised and that your doctor was not responsible for the injuries or complications associated with the hernia mesh.
-
- Take into consideration what the statute of limitations are– the deadlines until which you can file a lawsuit against the manufacturer. This is a complex legal question, and you should consult a lawyer or several lawyers on this topic. Don’t not call because you think you might not have a viable statute of limitations. Speak with one of our hernia mesh attorneys and let them decide if your hernia mesh claim can still be filed.
Speak with a lawyer experienced in handling hernia mesh cases, who can guide you through the complex and lengthy process with confidence and security. Get in touch with our law firm today so that we can review the specifics of your hernia mesh case and advise you on whether you have a valid hernia mesh claim against Covidien or any other hernia mesh defendant.

When Should I Expect My Covidien Hernia Mesh Lawsuit to Settle?
We cannot give a clear-cut answer regarding this matter. The timeline for a lawsuit can vary significantly depending on several factors, such as the complexity of the cases, the number of parties involved, the availability of relevant evidence, and the willingness of the opposing parties to negotiate and reach a compromise.
Many large litigations do not begin to settle until after bellwether trials take place. A bellwether trial is used to help determine the potential outcomes of similar cases that are pending and they are useful in helping to resolve large groups of similar cases. It typically takes several years of litigation before a bellwether trial takes place. If a bellwether trial results in a favorable outcome for the plaintiff, it may increase the likelihood that other plaintiffs will also be successful in their hernia mesh cases. We are still awaiting the fixed date of the initial bellwether trial in the Covidien hernia mesh litigations.
Any discussion of hernia mesh settlements are also highly confidential matters. Our hernia mesh attorneys will not be able to give hernia mesh settlement updates online. If and when hernia mesh settlements occur, our hernia mesh lawyers will be in contact by phone to give hernia mesh settlement updates and what to expect going forward.

What Financial Outcome Can I Expect From My Covidien Hernia Mesh Lawsuit?
It is difficult to predict the outcome, much less the financial outcome of a lawsuit involving a defective hernia mesh product manufactured by Covidien, especially because no bellwether trial has been conducted yet.
Even though the Covidien hernia mesh cases are consolidated in state and federal courts, each plaintiff is still an individual case. Therefore, the amount of money awarded to a Covidien hernia mesh plaintiff depends on the severity of the injuries or complications suffered, the clearness of a mesh defect that can be linked to the complications suffered, the cost of medical treatment and other damages, and the laws that are applicable in each state.
The plaintiffs may also be entitled to recover damages for non-economic losses, such as pain and suffering, or even the loss of a loved one. An experienced hernia mesh lawyer has to review the specifics and they might be able to advise you on the potential financial outcome of your hernia mesh case.
Contact Us Today to File a Covidien Hernia Mesh Lawsuit
If you or a loved one suffered from a defective Covidien hernia medical device, contact Nigh Goldenberg Raso & Vaughn to claim justice together today. There are no upfront fees– we are available for a free consultation to discuss your case and take necessary action. Call 202-792-7927 today for a free hernia mesh lawsuit consultation.
Lawsuits Against Other Hernia Mesh Manufacturers
For more information on the state and federal litigations against Bard, please visit our Bard hernia mesh lawsuit page. For general hernia mesh information, visit our hernia mesh lawsuit page.