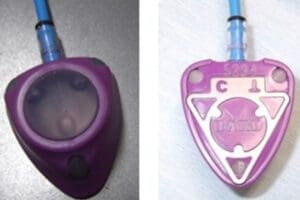
Synovo Total Hip Resurfacing System Recall.
The recent Synovo Total Hip Resurfacing System Recall has sent shockwaves through the medical community, raising urgent questions about patient safety and corporate accountability, with numerous lawsuits being filed in response. As legal challenges mount, those affected seek reliable information and professional guidance. If this recall has impacted you, it’s crucial to understand your rights and options. Nigh Goldenberg Raso & Vaughn stands ready to assist. Our experienced legal team specializes in handling complex medical device lawsuits and is committed to ensuring you receive the compensation and justice you deserve.
Company Background
Synovo Production, Inc. is a medical equipment company in Fullerton, California. and is the manufacturer of the Synovo Total Hip Resurfacing System. On March 23, 2023, the FDA issued an enforcement action against Synovo Production, Inc. for failing to document management reviews and quality audits. On January 3rd, 2024, the FDA warned against using the Synovo Total Hip Resurfacing System.
Detailed Description of the Recalled Device and Nature of the Defect.
The Synovo Total Hip Resurfacing System is a complex device comprised of several key components, including the Femoral Resurfacing Cup, the Acetabular Fixation Cup, and the Acetabular Bearing. Each element plays a critical role in the overall functioning of the device.
The defect in the Synovo Total Hip Resurfacing System lies in the significant modifications made to the Femoral Resurfacing Cup, the Acetabular Fixation Cup, and the Acetabular Bearing. These alterations have affected the device’s performance, leading to the recall.
The Synovo Total Hip Resurfacing System Recall was initiated in response to significant modifications made to the Femoral Resurfacing Cup, the Acetabular Fixation Cup, and the Acetabular Bearing, which are critical components of the device. The FDA did not approve these alterations.
It is important to note that “If your hip implant is functioning and you have no pain or worsening symptoms, the FDA does not recommend surgery to remove this device. Please continue the follow-up schedule recommended by your health care provider.”
Regulatory Response and the possibility of legal repercussions.
The U.S. Food and Drug Administration (FDA) has issued safety communications and warnings in response to the Synovo Total Hip Resurfacing System Recall. The risks identified by the regulatory body have further triggered concern among patients and the medical community.
Individuals affected by the Synovo Recall can seek legal help to file a Synovo Total Hip Resurfacing System Lawsuit. Nigh Goldenberg Raso & Vaughn is ready to help you navigate your potential claim.
The FDA has issued safety communications and warnings regarding the Synovo Total Hip Resurfacing System Recall. The regulatory body has identified several risks associated with the modified devices, triggering concern among patients and the medical community.
And they have issued the following recommendations for Healthcare providers:
- Do not purchase or implant the currently available Synovo Total Hip System.
- Remove from inventory, all Synovo Total Hip Systems, including the Femoral Resurfacing Cup, the Acetabular Fixation Cup, and the Acetabular Bearing components used in the system.
- Based on currently available information, the FDA does not recommend removal of Synovo Total Hip Systems from patients who do not have any new or worsening pain or symptoms.
- Be aware that safety and effectiveness have not been established for the Femoral Resurfacing Cup, the Acetabular Fixation Cup, and the Acetabular Bearing used in the Synovo Total Hip System.
- Discuss with patients considering hip implants the benefits and risks of all relevant treatment options, including alternative legally marketed hip implant devices.
- Closely monitor patients who have the Synovo Total Hip System, including resurfacing implants, for potential bone loss or device loosening, wear or failure. Consider obtaining X-rays to further evaluate a patient and their implanted device if you suspect device failure.
- Review the above “Recommendations for Patients” with patients who have received any components of the Synovo Total Hip System.
Understanding the Risks: Side Effects of Synovo Hip Replacement Products
Recent findings have raised concerns about the side effects associated with modified Synovo hip replacement products. These side effects vary in severity, and in some instances, patients may require additional surgery to remove or replace the device. It’s crucial for patients and healthcare providers to be aware of these potential risks.
Potential Side Effects of Synovo Hip Replacement Products:
- Bone Loss Over Time: Gradual deterioration of bone tissue surrounding the implant.
- Device Loosening: The implant may become unstable within the hip joint.
- Difficulty Bearing Weight: Challenges in standing or walking due to the implanted device
- Grinding Noises During Movement: Unusual sounds from the hip area during motion.
- New or Worsening Pain: Increased discomfort or pain in the hip area post-surgery.
- Weakness in the Hip Area: Reduced strength or stability in the hip region.
If you underwent a total hip replacement using Synovo products after 2019 and are experiencing any of these symptoms, it’s imperative to seek medical advice immediately. For those not currently experiencing side effects, regular monitoring by a healthcare provider is recommended to ensure the ongoing safety and effectiveness of the device.
Lawsuits Following the Recall
Following the Synovo Recall, numerous lawsuits have been filed against Synovo Production, Inc. Lack of patient communication regarding the recall has been a significant contributing factor to the number of lawsuits.
The lawsuits following the Synovo Recall might result in Multidistrict Litigation (MDL), which is a special federal legal procedure designed to speed up the process of handling complex cases.
Seeking Legal Help and Compensation
Patients affected by the Synovo Total Hip Resurfacing System recall have a critical opportunity to seek justice and compensation. If you or someone you know has been impacted, it’s time to act. Contact Nigh Goldenberg Raso & Vaughn law firm today at 202-792-7927. Our skilled legal team is deeply experienced in handling Synovo recall lawsuits and is dedicated to guiding you through every step of the legal process. The potential for substantial compensation exists, and our attorneys will explain in detail how to file your lawsuit. We work on a contingency fee basis, ensuring that legal fees are only applied if and when you receive compensation for the harm caused by this defective medical device. Don’t miss your chance to seek the compensation you deserve. Reach out to our team for a free case evaluation, and let us assist you in navigating the complexities of the Synovo Total Hip Resurfacing System lawsuit.
Frequently Asked Questions
What is the Synovo Total Hip Resurfacing System?
The Synovo Total Hip Resurfacing System is a medical device used in hip replacement surgeries.
Why was the Synovo Total Hip Resurfacing System recalled?
The FDA issued a warning letter on January 3, 2024, urging healthcare providers not to use the Synovo Total Hip Resurfacing System. The FDA became aware of modifications made to the components in 2022, and after an inspection, it issued a warning letter to Synovo, listing several violations and instructing the company to “immediately stop” making the system. The safety and effectiveness of the system and its components have not been established
What should patients who received the Synovo Total Hip Resurfacing System do?
Patients who received the Synovo Total Hip Resurfacing System should consult their healthcare providers for guidance. The FDA advised healthcare providers to closely monitor patients with the Synovo system for potential bone loss, device loosening, wear, or failure
Is there a lawsuit related to the Synovo Total Hip Resurfacing System?
Yes, there are lawsuits related to the Synovo Total Hip Resurfacing System. If you or a loved one suffered side effects of a Synovo hip replacement, you may be eligible to file a lawsuit. It is advisable to contact us for a free consultation.