Suboxone Tooth Decay Lawsuit – MDL 3092
Approximately 1 in 500 adults with insurance have been prescribed a form of Suboxone, a drug intended to primarily treat opioid use disorder. In late 2023, attorneys began filing Suboxone lawsuits. The Suboxone attorneys have sued the manufacturer of the drug Suboxone, Indivior, claiming that the pharmaceutical company failed to properly warn that Suboxone film would rot a user’s teeth. In early 2024, due to the number of Suboxone lawsuits on file around the country, the Suboxone lawsuits were all consolidated into MDL 3092. Since the formation of the Suboxone MDL, approximately 10,500 Suboxone dental lawsuits have been filed. Our Suboxone attorneys expect that several thousand additional Suboxone lawsuits will be filed within the next year. For a free Suboxone case evaluation, call 202-792-7927.
Suboxone Lawsuit MDL 3092 Timeline
June 2024 – Establishing Leadership Development Committee & Common Benefit
On June 14, 2024, the Suboxone plaintiff attorneys filed approximately 10,000 Suboxone lawsuits in a single day, which are listed in Schedule A per Suboxone case management order #4. The judge allowed the mass filing of Suboxone lawsuits on Schedule A, in part because pharmacy records requests are still pending for these Suboxone plaintiffs. Only plaintiffs who took name-brand Suboxone will be eligible to maintain a Suboxone lawsuit. Many of the 10,000 lawsuits that were filed on Schedule A will likely eventually be dismissed due to only taking generic Suboxone (The US Supreme Court has previously held that generic drug manufacturers are immune to liability from failing to adequately warn when their label matches the brand-name drug label).
On June 11, 2024, the Court entered Suboxone case management order #8 – Protocol for common-benefit fees and expenses. The Court noted that “Common-Benefit Work” includes all work done and expenses incurred that are of value to MDL 3092. The 20 page order details exactly what can be billed as common benefit work for the Suboxone MDL.
On June 6, 2024, the Court entered Suboxone case management order #7, setting forth the procedure to appoint a Suboxone Plaintiffs’ Leadership Development Committee (LDC). The Court noted that the Suboxone LDC is intended to provide mentorship to attorneys committed to long-term involvement in MDL practice with the goal of developing future MDL leaders. Attorneys wishing to be part of the Suboxone Film Leadership Development Committee have until July 8, 2024, to submit their applications.
May 2024 – Suboxone Document Production
The Court entered CMO 6 on May 21, 2024, which covered Evidence Rule 502(D) (Attorney-Client Privilege & Work Product) & other privileged materials. The Suboxone order notes that it will provide the maximum protection allowed under the Federal Rules of Evidence and shall be enforceable in all other State and federal proceedings. The Court order goes on to describe the process of claiming privilege and what must be included in a Suboxone privilege log.
On May 21, 2024, the Court also entered case management order #5, titled “Designation and Handling of Protected Information.” This court order governs the handling of Suboxone documents, depositions, deposition exhibits, interrogatory responses, responses to admissions, responses to request for production of documents, and all other Suboxone discovery obtained during the course of the Suboxone lawsuits. The lengthy court order also details how Suboxone documents can be classified as “Confidential Information” if the Suboxone documents contain:
- Information protected from disclosure by any applicable state or federal statute or regulation;
- Research, design, development, financial, technical, marketing, planning, regulatory, manufacturing, or commercial information that the Designating Party has maintained as confidential;
- Trade secrets; or
- any Protected Data.
The Court order then goes on the allow certain Suboxone documents to be labeled “Highly Confidential Information” if the information would create a substantial risk of competitive or business injury.
Who are allowed to view the confidential Suboxone documents that are produced in the Suboxone lawsuits?
- The Court, including attorneys, employees, judges, magistrates, secretaries, special masters, stenographic reporters, staff, transcribers, and all other personnel necessary to assist the Court in its function, and the jury (and any appellate court or other court);
- Mediators or other individuals engaged or consulted in Suboxone settlement negotiations on behalf of part of all of MDL 3092;
- Court reporters, stenographers, and videographers retained to record testimony taken in MDL 3092
- Suboxone attorneys and their employees who have responsibility for the preparation of Suboxone depositions and the Suboxone trial;
- Suboxone experts, consultants, investigators, or expert consulting firms retained by the Suboxone lawyers;
- Witnesses during their Suboxone depositions;
- Suboxone mock jurors or professional jury or trial consultants
On May 16, 2024, the Court entered Suboxone CMO 4 titled “Schedule A Complaint”. The order notes that the Suboxone Plaintiffs’ Leadership had requested a tolling agreement to afford counsel time to investigate their Suboxone claims by, for example, obtaining medical or pharmacy records to substantiate Suboxone use. The Suboxone Plaintiffs’ leadership was concerned that a flood of Suboxone lawsuits might be filed without adequate vetting to demonstrate that the plaintiff actually took name brand Suboxone in order to protect potential statutes of limitations triggered by a warning change to the Suboxone label in June of 2022. The Suboxone defendants refused to agree to tolling, so the judge ordered that the Suboxone plaintiffs could file a complaint on the master Suboxone MDL docket captioned “The Individuals Identified on Schedule A v. Individor, Inc. et al.” The following information must be included for each Suboxone lawsuit filed via Schedule A:
- Suboxone Plaintiff’s first and last name;
- First and Last name of any consortium Suboxone Plaintiff;
- Law Firm representing each Suboxone Plaintiff;
- Location where Suboxone Plaintiff is a resident and citizen;
- Suboxone Plaintiff’s designated venue;
- Location (city, State) where Suboxone Plaintiff currently resides;
- Location(s) (city, State) where Suboxone Plaintiff claims, alleges, or represents they were prescribed Suboxone film; and
- Location(s) (city, State) where Suboxone Plaintiff claims, alleges, or represents they used Suboxone film.
Following the filing of Suboxone lawsuits via Schedule A, the Suboxone defendants will have until July 1, 2024, to file a motion to sever and the Court will then set a briefing schedule with the expectation that Suboxone lawsuits filed on Schedule A will then file separate individual actions, which will be a continuation of the original suit filed via Schedule A.
March 2024 – Initial Suboxone Status Conference
As per case management order 1 of the Suboxone litigation, the first case management conference was held on March 7, 2024. Following the first case management conference, the Judge appointed Plaintiffs’ leadership for MDL 3092 in the 2nd Suboxone case management order on March 8, 2024. A direct filing order allowing all Suboxone lawsuits nationwide to be filed into MDL 3092 was entered on March 18, 2024, and later amended on March 27, 2024. Lawsuits filed into an MDL typically greatly increase once a direct filing order is entered.

February 2024 – Suboxone MDL 3092 Created
On February 2, 2024, the Judicial Panel on MultiDistrict Litigation (JPML) issued a transfer order to centralize all Suboxone lawsuits into the Northern District of Ohio under Judge Philip Calabrese. At the time the JPML consolidated, there were 15 Suboxone lawsuits on file in 5 different federal district courts. The JPML held that consolidating the Suboxone lawsuits “in the Northern District of Ohio will serve the convenience of the parties and witnesses and promote the just and efficient conduct of this litigation.” The Suboxone defendants had objected to consolidation and the JPML noted, “defendant’s objection is not taken well. Centralization allows for streamlined discovery, briefing, and rulings regarding personal jurisdiction of the Reckitt defendants before a single judge.” In regard to having Judge Philip Calabrese preside over the Suboxone dental lawsuits, the JPML commented that they were able to “assign this litigation to a jurist who has not yet had the opportunity to preside over an MDL.” The JPML gave MDL 3092 the official title “In re: Suboxone (Buprenorphine/Naloxone) Film Products Liability Litigation.”
Suboxone Settlements & Judgments
October 2023 – $385 Million Suboxone Settlement
On October 23, 2023, a $385 million Suboxone settlement with direct purchasers of Suboxone was announced to resolve the Suboxone antitrust Multi-District Litigation (MDL), which was pending in the United States District Court for the Eastern District of Pennsylvania. It was noted that “the $385 million is expected to be made in November 2023 and funded from Indivior’s (Suboxone’s manufacturer) existing cash.” It was further noted that the Suboxone antitrust MDL had been litigated for a decade.
April 2021 – $300 Million Suboxone Settlement
In April of 2021, the makers of Suboxone agreed to a $300 million settlement for falsely and aggressively marketing and promoting Suboxone, resulting in improper expenditures of state Medicaid funds. The Attorney General of Maryland commented that the makers of Suboxone “misled the public about Suboxone’s risks, promoted its use to physicians, and defrauded state Medicaid programs out of hundreds of millions of dollars to increase its bottom line.” It was also noted that the Suboxone settlement resolved six whistleblower lawsuits pending in federal courts in Virginia and New Jersey.

November 2020 – $289 Million Suboxone Criminal Penalty
On November 12, 2020, it was announced that the manufacturer of Suboxone would pay $289 million in criminal penalties in connection with a guilty plea for improperly marketing the Suboxone. U.S. District Judge James P. Jones of the Western District of Virginia entered the sentence against the maker of Suboxone pursuant to a plea agreement. The Acting Assistant Attorney General of the U.S. Justice Department’s Civil Division commented, “We will hold drug manufacturers accountable when they make representations that could affect consumer’s access to opioid addiction treatment.”
October 2020 – Suboxone CEO Sentenced to Jail

The CEOs of pharmaceutical companies are rarely held accountable for their company’s bad behavior. However, that wasn’t the case when it came to the CEO of the company that manufactures Suboxone. On October 22, 2022, Shaun Thaxter, the former CEO of the company (Indivior) that manufactures Suboxone, was sentenced to 6 months in federal prison and ordered to pay a $100,000 fine and forfeit $500,000. A United States Attorney noted, “Thaxter failed to prevent efforts to build profits through misleading safety claims, which led to millions of dollars in ill-gotten gains for Individor. As the Court recognized today, this sentence should serve as a deterrence to other pharmaceutical executives.” According to court documents, the Suboxone CEO had authority over the marketing and sales of Suboxone Film which, along with other Suboxone products, generated nearly all of the company’s revenue. Furthermore, in 2012, the CEO oversaw and encouraged Individor’s efforts to secure formulary coverage for Suboxone Film from the Massachusetts Medicaid agency called MassHealth. The CEO also asked employees to devise a strategy to win preferred drug status for Suboxone Film and counteract a non-opioid competitor MassHealth was considering for opioid addiction treatment. Some employees had even shared false and misleading information with MassHealth about Suboxone Film’s risk of accidental pediatric exposure. Just two months after receiving the false information, MassHealth announced it would approve Suboxone Film for Medicaid patients with children under the age of six.
June 2020 – Suboxone Manufacturer CEO Pleads Guilty
On June 30, 2020, Shaun Thaxter, CEO of Indivior, pleaded guilty to causing the introduction of misbranded Suboxone Film into interstate commerce in violation of the Federal Food, Drug, and Cosmetic Act. Thaxter had been the CEO of the companies that manufactured Suboxone (Individor – formerly Reckitt Benckiser Pharma). The Suboxone Film was considered misbranded due to untruthful drug safety claims. The Suboxone CEO is scheduled to be sentenced in U.S. District Court in Abingdon in October of 2020.

FDA Warns Suboxone Film Can Cause Tooth Decay
On January 12, 2022, the FDA issued a Drug Safety Communication regarding Suboxone Film and other buprenorphine medications that dissolve in the mouth causing dental problems. The FDA specifically warned that Suboxone film can cause “dental problems, including tooth decay, cavities, oral infections, and loss of teeth, can be serious and have been reported even in patients with no history of dental issues.”
The FDA also issued a warning to health care professionals at the same time, warning that Suboxone film could cause “cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss. Multiple cases were reported in patients with no prior history of dental problems. The most common treatment for the dental adverse events was tooth extraction/removal.”
Why Did The FDA Warn Suboxone Film Can Cause Tooth Decay?
The FDA found over 300 cases of dental problems, nearly half of which were classified as serious dental problems, in users who had taken Suboxone film or similar medications dissolved in the mouth. The FDA noted that there are likely additional instances of Suboxone film causing dental problems, as the FDA was only aware of reports directly submitted to the FDA or published in the medical literature. As most patients and medical providers would expect Suboxone film to cause tooth decay, the number of individuals impacted is likely significantly higher. Our Suboxone lawyers suspect that 10’s of thousands of people in the United States have experienced dental complications as a result of Suboxone film and similar medications.
The FDA noted that the average patient age for Suboxone film users who were experiencing tooth decay was 42 years old. However, the FDA also saw evidence of those as young as 18 experiencing dental damage as a result of Suboxone film. Most Suboxone film users experiencing dental problems were prescribed Suboxone film for opioid use disorder, while about 10% were prescribed to treat chronic pain.
In less than 10% of cases that the FDA was aware of, the Suboxone film user had no prior history of dental problems. Interestingly, the FDA found that dental problems could occur as soon as 2 weeks after starting Suboxone film, with the average time to diagnosis being 2 years after starting treatment. In approximately 33% of cases, numerous teeth were impacted by Suboxone film. The most common treatments for these dental problems were tooth extraction and tooth removal, which occurred in about 25% of cases.
The FDA seemed very confident in its Suboxone film dental safety advisory communication because most of the cases that had been reported to the FDA were by health care professionals and included extensive documentation and medical records demonstrating the progression of the dental adverse events.
What is the FDA doing about Suboxone Film?
Not a lot – the FDA stated in its January 2022 Suboxone Film safety communication that it would be requiring a new warning about the risk of dental problems to be added to the prescribing information and patient Medication Guide. The FDA noted that the prescribing and patient information would also be updated to include strategies to maintain or improve oral health while using Suboxone film. However, these “strategies” merely “include recommending that prescribers refer patients to dental care services and encourage them to have regular checkups while taking [Suboxone film].”
Suboxone Film Instructions For Use Warnings Updated

On June 17, 2022, approximately 6 months after the FDA said that it was going to require the Suboxone film instructions for use (IFU) to include a warning regarding the association of Suboxone film and dental damage, was the IFU finally updated to include a warning. It’s unknown at this time why it took so long for any warning at all to finally appear in the IFU for Suboxone film. What is obvious though is that the warning that was finally added to the Suboxone film instructions for use is an incredibly weak warning that was unlikely to put any patient or treater on notice that Suboxone film can cause tooth decay. There is no boxed warning (formerly known as Black Box Warning), which is the highest safety-related warning, that Suboxone film can cause dental damage or tooth decay. A boxed warning is appropriate when there is reasonable evidence of an association of a serious hazard from a drug. After the FDA’s January 2022 safety communication on Suboxone film, it is perplexing why a boxed warning regarding dental damage was not required.
Even more perplexing, is why no warning regarding dental damage was added anywhere on the first page of the updated Suboxone film IFU. The first page of the IFU for Suboxone film has a long list of warnings and precautions including, addiction, abuse, misuse, respiratory depression, unintentional pediatric exposure, neonatal opioid withdrawal syndrome, adrenal insufficiency, risk of opioid withdrawal, risk of hepatitis, and risk of overdose. Why doesn’t the company just add that there is a risk of dental injuries and tooth decay on the front page warnings on the Suboxone film IFU? Probably because prominently warning of severe dental damage and tooth decay would result in less sales of Suboxone film and ultimately less profit for the company. Also, the FDA being well aware of the association between Suboxone film and dental damage, yet not requiring a warning on the first page of the Suboxone film IFU begs the question – Who is the FDA actually protecting?
Suboxone Film Method of Administration Updated
The first update to the Suboxone film instructions for use appears on page 5 and does not contain any type of warning that Suboxone film can cause dental damage or tooth decay. Instead of adding a prominent warning regarding tooth decay, the manufacturers of Suboxone film added the following to the “Method of Administration – Buccal Administration” section of the IFU:
“Advise patients to do the following after the product has completely dissolved in the oral mucosa: take a sip of water, swish gently around the teeth and gums, and swallow. Advise patients to wait for at least one hour after taking SUBOXONE before brushing teeth.”
However, the instructions for use are completely silent on why a patient should rinse their mouth out with water after taking Suboxone film and why the patient shouldn’t brush their teeth for at least one hour after taking Suboxone film. Patients and physicians might be much more concerned if the instructions for use disclosed that if you brush your teeth within an hour of taking Suboxone film, you could brush the enamel (protective coating) off of your teeth, or that patients need to rinse their mouth out with water after taking Suboxone film because it is so acidic that it will rot the user’s teeth out if they don’t rinse their mouth. It is our Suboxone attorneys’ opinion that such important instructions regarding the need to rinse one’s teeth and not brush one’s teeth after taking Suboxone film should appear on the first page of the instructions for use.
Suboxone Film Adds Hidden Dental Damage Warning to IFU
The full Warnings and Precautions section of the Suboxone film instructions for use starts on page 7. Not until the bottom of page 10 does the Suboxone film IFU disclose “Dental Adverse Events”. The entire section on dental adverse events related to Suboxone film in the IFU reads as follows:
“Cases of dental caries, some severe (i.e., tooth fracture, tooth loss), have been reported following the use of transmucosal buprenorphine-containing products. Reported events include cavities, tooth decay, dental abscesses/infection, rampant caries, tooth erosion, fillings falling out, and in some cases, total tooth loss. Treatment for these events included tooth extraction, root canal, dental surgery, as well as other restorative procedures (i.e., fillings, crowns, implants, dentures). Multiple cases were reported in individuals without any prior history of dental problems. Refer patients to dental care services and encourage them to have regular dental checkups while taking SUBOXONE. Educate patients to seek dental care strategies to maintain or improve oral health while being treated with transmucosal buprenorphine-containing products. Strategies include, but are not limited to, gently rinsing the teeth and gums with water and then swallowing after SUBOXONE has been completely dissolved in the oral mucosa. Advise patients to wait for at least one hour after taking SUBOXONE before brushing teeth.”
It is our Suboxone lawyers’ opinion that the warnings regarding dental damage and tooth decay should appear on the first page of the Instructions of Use, preferably in a boxed warning. The causative association between Suboxone film and tooth decay is clear. Furthermore, tens of thousands of patients nationwide have alleged that they are victims of Suboxone film causing severe tooth decay and dental damage. There is no valid reason to not display a prominent warning to potential users and treaters that Suboxone film can cause dental damage.
Warning Update Did Not Put Suboxone Users On Notice
Our Suboxone lawyers believe that the warning update in the instructions for use was insufficient to put users on notice that Suboxone film caused their teeth to rot out. No dear doctor letter was sent out notifying physicians of the newly acknowledged link between Suboxone film and dental damage. Similarly, no dear patient letter was sent out of Suboxone users letting them know that Suboxone film could have damaged their teeth. A long-term Suboxone film user would have had no reason to randomly go to page 10 of the instructions for use after June of 2022. Also, anyone who stopped taking Suboxone film prior to June 2022 would never have even received an updated instructions for use. For most Suboxone film users, the first time they learned of an association between Suboxone film and dental damage was when they saw an attorney advertisement. Because of this, our Suboxone lawyers believe that the statute of limitations for a Suboxone lawsuit hasn’t expired. Regardless of what state you live in, if you’ve experienced tooth decay after taking name-brand Suboxone film, call our Suboxone attorneys today at 202-792-7927.
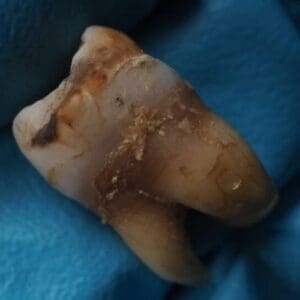
Suboxone Film Dental Injuries
Suboxone has been linked to severe dental issues, leading to multiple allegations and lawsuits. Individuals have reported a variety of dental problems, from cavities and tooth decay to more severe conditions such as tooth loss and oral infections. These issues allegedly stem from the acidity of buprenorphine, a key component of Suboxone, which can damage tooth enamel and overall dental health.
-
- Cavities and tooth decay
-
- Cracked teeth and the need for crown or crown replacements
-
- Oral infections
-
- Loss of tooth enamel
-
- Necessity for root canal treatments
-
- Complete tooth loss
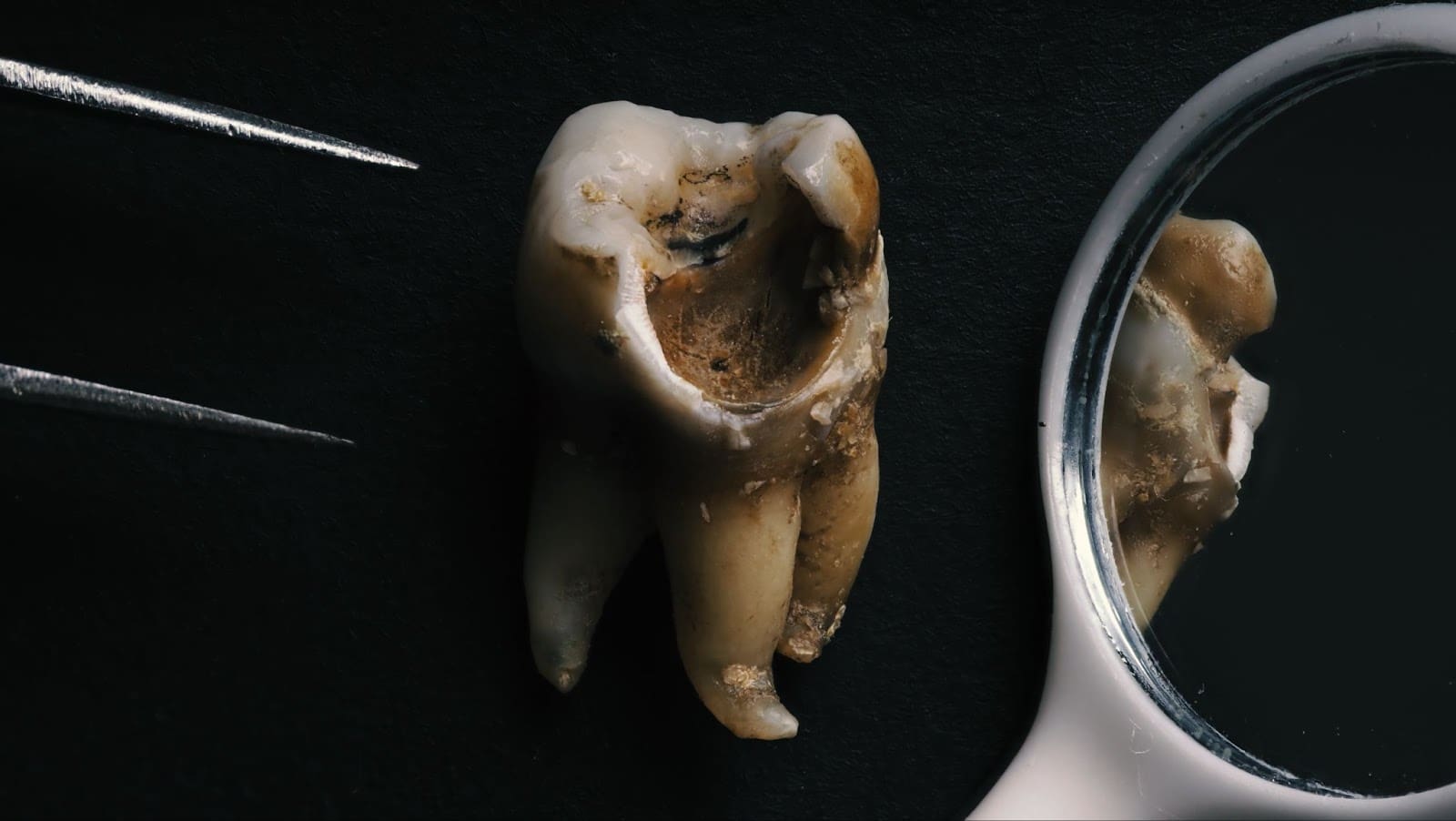
The Science Behind Suboxone and Dental Issues
Suboxone, a medication primarily used in opioid addiction treatment, is administered sublingually, meaning it dissolves under the tongue. This method, while effective for drug delivery, exposes the teeth and gums to the medication’s active ingredients, notably buprenorphine. The acidity of buprenorphine is a contributing factor to dental issues such as tooth decay, cavities, and even tooth loss.
Not all individuals taking Suboxone will experience dental problems; however, those who do should not only seek immediate dental intervention but also consider legal consultation. Legal professionals specializing in pharmaceutical litigation can provide guidance on potential compensation claims related to Suboxone-induced dental issues. As the legal landscape surrounding Suboxone continues to evolve, staying informed about both medical and legal developments is crucial for patients and healthcare practitioners alike.
Suboxone FAQs
When Will I Get A Suboxone Settlement Check?
Many of our Suboxone clients ask us when they will be receiving a settlement check, as they would like to get their teeth fixed as soon as possible. Unfortunately, that is impossible to answer at this time as there has not yet been a Suboxone settlement related to personal injury claims, such as for tooth decay and other dental injuries. The Suboxone dental personal injury claims are currently in the process of being litigated. The Suboxone dental lawsuits were only consolidated a few months ago. In terms of a mass personal injury lawsuit against a pharmaceutical company, the Suboxone tooth decay litigation is still in its infancy. Most mass litigations against a pharmaceutical company for personal injury claims take many years of litigation before a settlement is reached. There is also typically a claims administration process to properly allocate the money to each plaintiff based on injury severity, causation, and other factors. The claims administration process itself can take around 1 year. Simply put – personal injury litigation against a pharmaceutical company is a lengthy process. Don’t delay in contacting our Suboxone attorneys though, as our Suboxone lawyers need time to get supporting medical records and protect potentially applicable statutes of limitations related to your Suboxone dental injuries.
What compensation can be expected from a Suboxone lawsuit?
The Suboxone lawsuits are seeking to achieve financial compensation for those who used name-brand Suboxone Film and then suffered complications with their teeth, such as tooth decay, teeth falling out, teeth needing extracted, infections and other dental-related injuries. Because the Suboxone dental damage lawsuits are personal injury claims, the amount of compensation received through any Suboxone settlement will vary significantly based on the facts specific to each Suboxone user’s lawsuit. The severity of one’s dental injuries, the degree of related medical treatment, other risk factors that could have caused dental damage, prior dental history, and various applicable state laws will likely all impact how much someone will receive if a Suboxone settlement is eventually reached for dental injuries.
Do you have to have taken name-brand Suboxone to qualify for a Suboxone lawsuit?
Yes, only those who took name-brand Suboxone Film qualify for a Suboxone lawsuit. Those who only took generic Suboxone Film (buprenorphine/naloxone) do not qualify for a Suboxone lawsuit.
Why Don’t Generic Suboxone Film Users Qualify for a Suboxone Lawsuit?
The Suboxone Film tooth decay lawsuits allege that the manufacturer failed to warn that Suboxone Film can cause dental damage. Per FDA regulations, a generic drug manufacturer must have the same warnings as the name-brand and the generic drug manufacturer is unable to change the label. Therefore, the US Supreme Court in PLIVA v. Mensing previously held that a generic drug manufacturer can not be sued for failure to warn if their label matches that of the name-brand drug.
What impact does Suboxone have on dental health?
The FDA issued a warning that sublingual medications containing buprenorphine, such as Suboxone Film, have been linked to various dental issues. The buprenorphine in Suboxone Film is very acidic and will weaken the enamel of a user’s teeth. Users of Suboxone Film have reported to our Suboxone attorneys that they started experiencing oral infections, significant tooth decay, cavities, teeth cracking, and numerous teeth either falling out on their own or needing extraction. Some Suboxone Film users have even reported losing every tooth. The FDA also noted that dental damage was reported as soon as 2 weeks after starting Suboxone Film.
What Is Required to File a Suboxone Dental Damage Lawsuit?
In order for our Suboxone attorneys to file a Suboxone dental damage lawsuit on your behalf, the following requirements must be met:
-
- Must have taken name-brand Suboxone film (Suboxone Film went generic in June of 2018, so most users that started after June of 2018 will have taken generic Suboxone Film). Feel free to send photos if you still have the Suboxone Film packaging.
-
- Must have experienced dental complications after starting Suboxone Film.
Suboxone Tooth Decay Lawyer
If you or someone you know has suffered from tooth decay or other dental issues after taking Suboxone sublingual film, Contact us today for a free Suboxone lawsuit consultation. Let our Suboxone team at Nigh Goldenberg Raso & Vaughn assist you through every step of your legal journey. Call 202-792-7927 to talk to our Suboxone attorneys today.


