Recalled Exactech Knee Lawsuit
Exactech recalled 147,732 Exactech artificial knee implants on August 31, 2021, which has already led to hundreds of Exactech recall lawsuits being filed around the nation. An example of Exactech’s knee recall letter can be viewed here. Many patients report never receiving an Exactech knee recall letter, even though they were implanted with a recalled Exactech knee replacement device.
Exactech is headquartered in Gainsville, Florida. As a result, lawsuits are being filed related to recalled Exactech knees in both Florida state court and federal courts around the nation. While Exactech is located in Florida, the Judicial Panel on Multidistrict Litigation (JPML) consolidated all recalled Exactech implants in the Eastern District of New York. The consolidated proceeding for recalled Exactech products is known as a Multidistrict Litigation or MDL. The MDL for recalled Exactech implants is in front of Judge Nicholas G. Garaufis. In addition to Exactech recalling its knee implants, Exactech also recalled its hip implants and ankle implants.
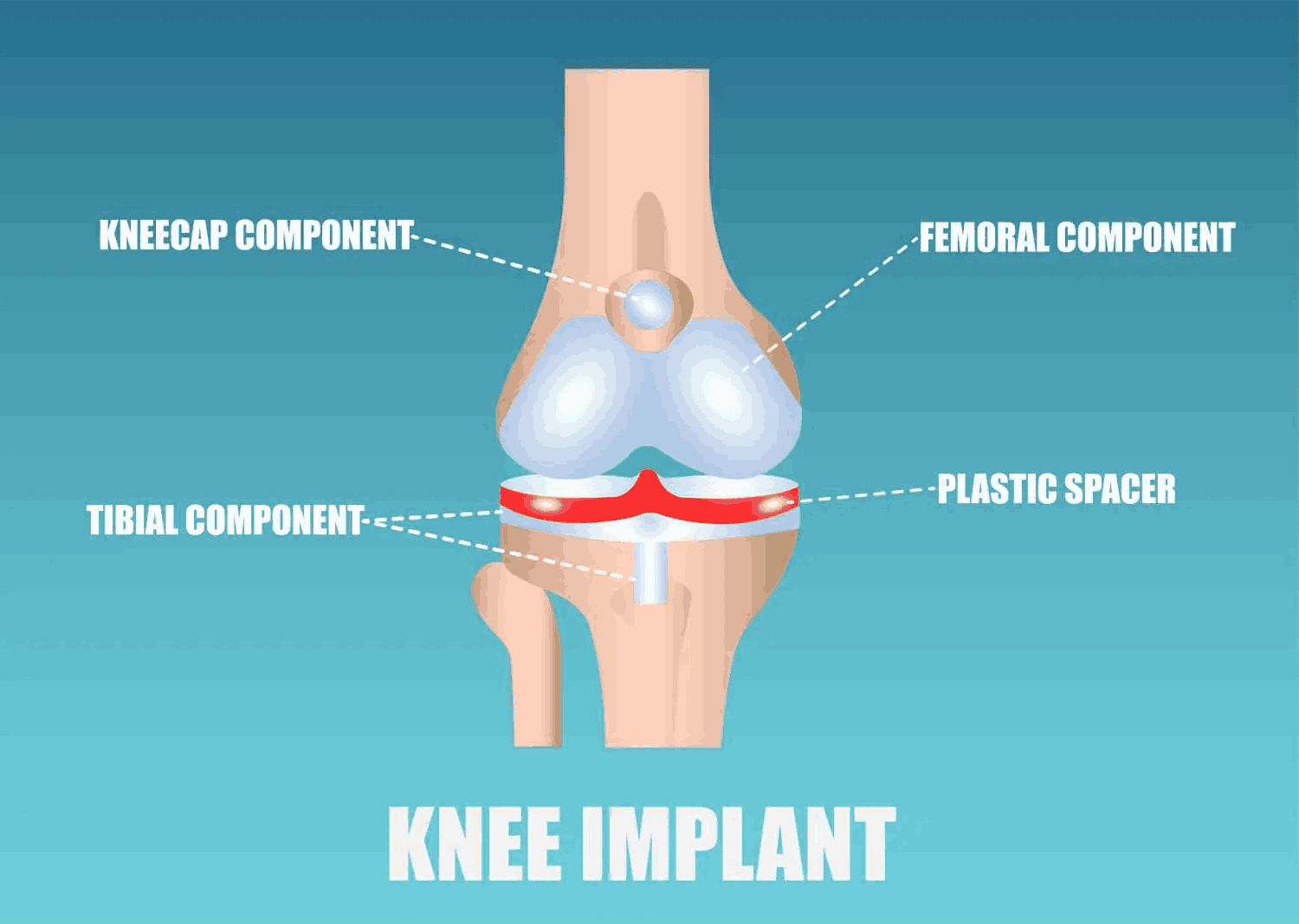
Exactech Knee Implant Components
The recalled Exactech knee has four main components:
- Kneecap Component– Also known as the patellar component. The kneecap component of the Exactech knee implant is made of plastic and fits on the kneecap (patella).
- Femoral Component– The femoral component of the Exactech knee is made of metal and attaches to the femur (upper leg).
- Tibial Component– The tibial component of the Exactech knee is a metal tray that fits into the tibia (lower leg).
- Plastic Spacer– The plastic spacer of the Exactech knee is made out of a plastic known as polyethylene and fits between the femoral component (upper leg) and the tibial component (lower leg). The Exactech plastic spacer is intended to mimic the cartilage of the knee and provide cushion.
Defective Exactech Knee Component – Plastic Liner
The Exactech knee was recalled due to defective plastic spacers. Exactech learned that the packaging for the tibial insert had been out of specification – allowing the plastic polyethylene spacer to be exposed to air. The oxygen in the air can degrade the Exactech plastic polyethylene spacer through a process known as oxidation. Oxidative degradation of the Exactech plastic polyethylene spacer can result in the Exactech knee failing earlier than anticipated. Oxygen has long been known to degrade polyethylene and Exactech should have been aware that their knee implants would fail early if their knee implants were prematurely exposed to oxygen.
FDA Safety Communication – Risk with Exactech Knee Replacement Devices
On March 23, 2023, the U.S. Food and Drug Administration (FDA) issued a statement reminding all patients and health care providers about Exactech knee replacement devices manufactured by Exactech between 2004 and August 2021, and recalled in 2021 and 2022. The FDA noted that many recalled “Exactech joint replacement devices (including knees, ankles, and hips) were packaged in defective packaging bags. These defective bags were missing one of the oxygen barrier layers that protect devices from oxidation, a chemical reaction with oxygen that can degrade plastics over time. Oxidation can lead to accelerated device wear/failure, and component cracking or fracture, all leading to corrective revision surgery. Some of the recalled devices are associated with increased risk of revision surgeries and bone loss related to excessive device wear/failure.”
Recalled Exactech Knee Lawsuit Attorney
If you or a loved one has been implanted with an Exactech knee and underwent revision surgery or received an Exactech knee recall letter, contact our Exactech knee attorneys today by filling out a web form or calling 202-792-7927. Time is limited to file a lawsuit related to the Exactech knee recall – contact our Exactech knee recall lawyers today. Compensation could be available for those who have been injured and experienced additional surgeries due to a recalled Exactech knee implant. To learn more about the Exactech recalls, visit our Exactech Lawsuit page.



