Hospital Bacterial Outbreaks
Hospital Superbug Outbreaks: 2026 Investigation & Litigation Updates Active investigations at Vanderbilt, Providence, and UCLA have linked routine procedures to life-threatening infections like CRE, Sepsis, and bloodborne pathogens. While manufacturers are often to blame, 2025-2026 findings suggest hospital cleaning breaches are also at fault. If you contracted a “superbug” or received a notice of exposure after a recent endoscopy, you may have a claim for medical negligence. Learn the signs of hospital-acquired infections and how new 2026 CDC safety mandates may affect your case.
Olympus Endoscope Recalls

Olympus Recall Alert: New January 2026 Warnings & Fatal Infection Risks Beyond the October 2025 “superbug” alerts, Olympus has expanded its 2026 recalls to include fatal component failures in ViziShot needles and high-risk insufflation units. With the FDA now blocking 58 models from U.S. entry, patients who suffered sepsis or injury after a 2024–2025 procedure may have a critical claim. Learn about the new 10X magnification standards and why the 2026 recall expansions are being called a “systemic failure” by legal experts.
Olympus Duodenoscope Infection Lawsuits

Olympus Duodenoscope Infections: October 2025 Safety Alert & Lawsuit Update A critical October 2025 safety notice has linked Olympus TJF-series duodenoscopes to ongoing “superbug” outbreaks and sepsis. Despite following sterilization protocols, patients are contracting drug-resistant infections due to a design defect in the device’s elevator mechanism. If you underwent an ERCP procedure and suffered a severe infection or organ failure, you may be eligible for the 2026 wave of litigation.
Spinal Cord Stimulator Lawsuit
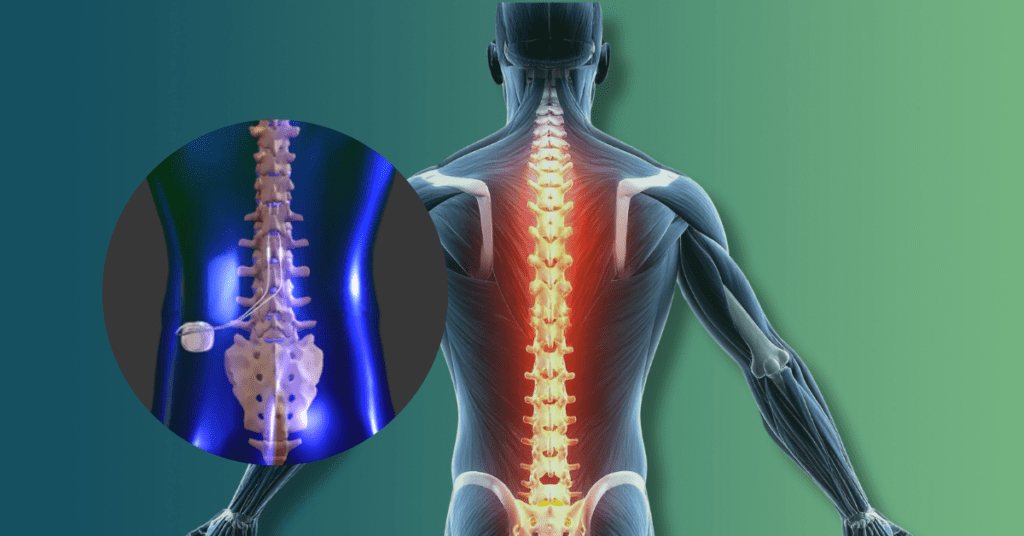
Spinal Cord Stimulators promised chronic pain relief but have left thousands with permanent injuries, including loss of bowel control and paralysis. If you required revision surgery for a device from Medtronic, Abbott, or Boston Scientific, you may be entitled to a settlement. Discover your legal options and get a free case review today.
Cartiva Toe Implant Lawsuit
Stay updated on the Cartiva Toe Implant lawsuit as the JPML moves to consolidate cases in 2026. With failure rates reported as high as 64% and a 2024 FDA recall in place, patients suffering from implant subsidence or seeking revision surgery may be eligible for compensation. Learn about the current case status and your legal rights.
AngioDynamics Port Catheter Lawsuit
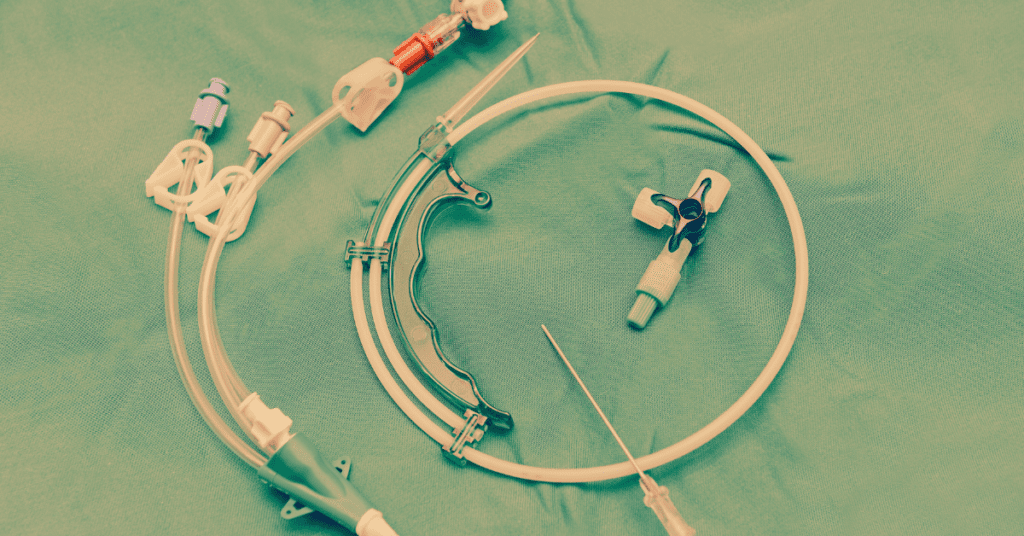
AngioDynamics Port Catheter Litigation (MDL 3125) Lawsuits filed nationwide allege that AngioDynamics port catheters—specifically the SmartPort, Vortex, and Xcela models—suffer from a critical design defect involving barium sulfate. This defect allegedly causes the catheter material to degrade, fracture, or migrate, leading to severe complications such as sepsis, blood clots, and organ perforation. Federal cases have been consolidated into Multidistrict Litigation (MDL No. 3125) in the Southern District of California.
BioZorb Breast Marker Lawsuit Filings
Stay up to date on the BioZorb Breast Marker Lawsuit and pending MDL proceedings. This page provides official court filings, Pretrial Orders (PTOs), and Case Management Orders (CMOs) related to claims that the BioZorb surgical marker caused chronic pain, infections, and disfigurement after breast surgery. Check back regularly for updates as the BioZorb lawsuits progress toward federal consolidation and coordinated discovery.
Gore-Tex Hernia Mesh Lawsuit
Gore-Tex DualMesh was marketed as a breakthrough in hernia repair, but patients have reported severe complications including chronic pain, infections, bowel obstruction, and mesh migration. Made from ePTFE (Teflon), the mesh’s shrinking and porosity can cause long-term injuries requiring surgical removal. Despite these risks, the FDA has not recalled Gore-Tex DualMesh. Attorney Brett Vaughn is currently one of the few lawyers nationwide pursuing cases for those harmed by this device.
Bard Hernia Mesh Lawsuit
Bard hernia mesh lawsuits are pending in Rhode Island state court and in federal court. Thousands of Bard hernia mesh lawsuits have been filed.
Covidien Hernia Mesh Lawsuit
Covidien Hernia Mesh Lawsuits Covidien is the second largest hernia mesh manufacturer in the world after Bard. In 2014, Covidien was acquired by Medtronic for $42.9 billion. Covidien is located in Massachusetts and Medtronic is located in Minnesota. Like most hernia mesh manufacturers in the United States, Covidien sells polypropylene-based hernia meshes. However, Covidien is […]