Spinal Cord Stimulator Lawsuit
Were You Injured by a Spinal Cord Stimulator?
Spinal cord stimulators (SCS) are implanted medical devices marketed as a solution for chronic pain. For many patients, however, these devices have caused devastating complications—including nerve damage, loss of bowel or bladder control, severe electrical burns, and worsening pain.
Nigh Goldenberg Raso & Vaughn (NGRV) is actively investigating and pursuing spinal cord stimulator lawsuits on behalf of individuals harmed by defective SCS devices manufactured by companies such as Abbott (St. Jude), Boston Scientific, Medtronic, and Nevro.
If you or a loved one experienced serious complications after receiving a spinal cord stimulator, you may be entitled to financial compensation.
Breaking Update: February 2026
New Abbott Eterna Lawsuits: As of January 27, 2026, new product liability claims (including Tuttle v. Abbott) have been filed regarding the Abbott Eterna device. Allegations focus on rapid lead migration and “unauthorized practice of medicine” by sales representatives.
Shock Episodes Documented: Recent filings are now utilizing device interrogation reports to prove unexplained “jolting” sensations and system resets, strengthening claims of manufacturing defects.
What Is a Spinal Cord Stimulator?
A spinal cord stimulator is an implanted device designed to deliver low-level electrical pulses to the spinal cord in an effort to block pain signals before they reach the brain. The system typically includes:
- Thin electrode leads placed in the epidural space near the spinal cord
- An implantable pulse generator (IPG), similar to a pacemaker battery
- A handheld remote control used by the patient to adjust stimulation levels
While these devices are promoted as safe and effective, mounting evidence shows that many spinal cord stimulators malfunction, overheat, or deliver dangerous electrical output—leading to severe and sometimes permanent injuries.
Why Are Lawsuits Being Filed?
Despite being marketed as safe, FDA data shows that between 2004 and 2019, there were nearly 180,000 adverse event reports related to Class III SCS devices, including over 118,000 injuries.
Lawsuits allege that manufacturers (Abbott, Boston Scientific, Medtronic, and Nevro) failed to warn patients and doctors about severe risks, including:
- Manufacturing Defects: Devices heating up beyond FDA-approved limits (over 42°C), causing internal burns.
- Failure to Report: Manufacturers allegedly failed to report adverse events to the FDA as required by law.
- Negligence & “Practicing Medicine”: Sales representatives—not doctors—are often the ones adjusting device settings (voltage/frequency) for patients, sometimes in casual settings like restaurants, which plaintiffs argue constitutes practicing medicine without a license.
- False Marketing: Sales reps promising patients they could resume physical activities that are actually impossible with the device, or falsely claiming the device would reduce pain by over 50%.
Serious Injuries & Complications
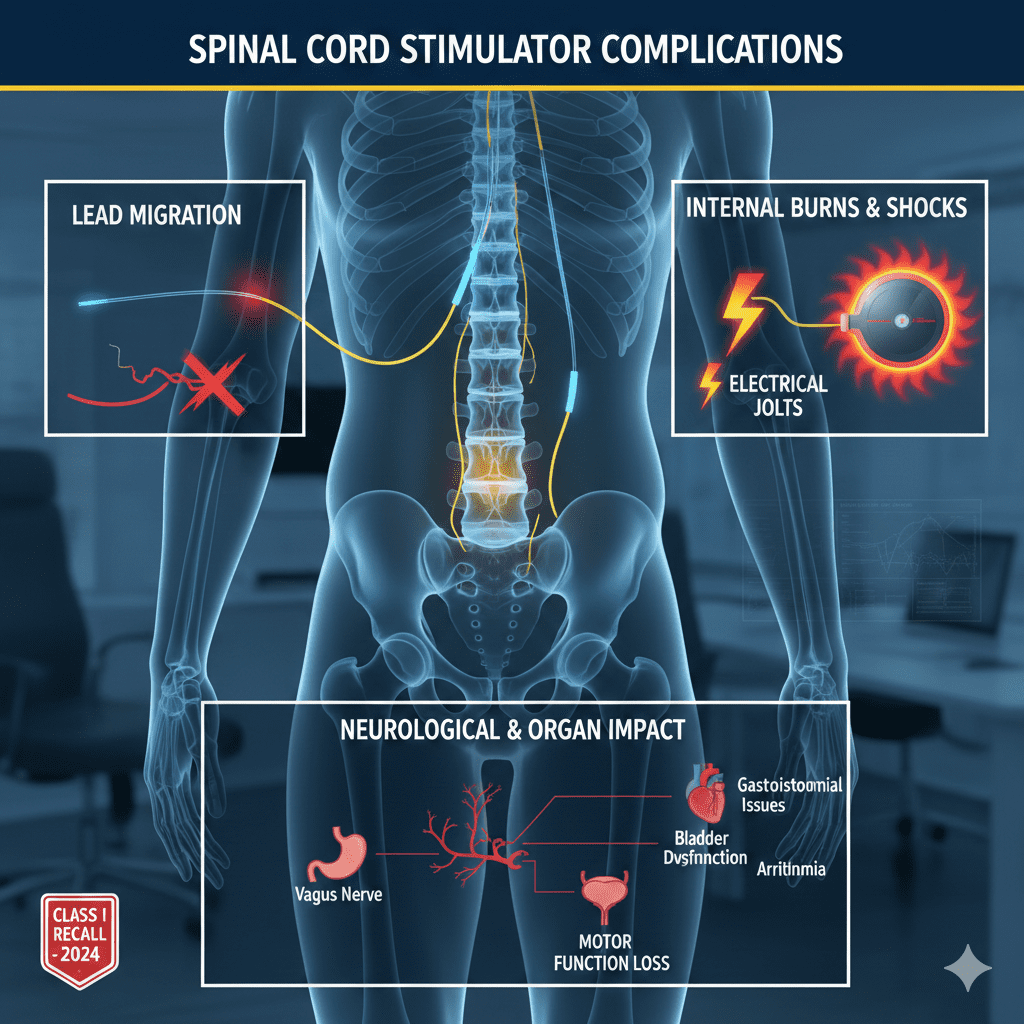
Our firm is investigating claims involving severe injuries caused by SCS devices. Common complications cited in lawsuits include:
1. Burns and Electrical Shocks
Defective batteries can overheat, causing internal tissue burns. Patients also report sudden, painful “jolts” or electrical shocks.
2. Device Migration and Lead Fracture
The leads (wires) implanted near the spine can move (migrate) or break. This often renders the device useless and requires invasive surgery to correct.
3. Internal Organ Damage (Vagus Nerve Impact)
Stray electrical impulses can affect the Vagus Nerve, which controls the parasympathetic nervous system. This can lead to surprising and debilitating side effects, including:
- Gastrointestinal Issues: Gastroparesis (stomach paralysis), chronic diarrhea, and fecal incontinence.
- Bladder Dysfunction: Urinary incontinence or retention.
- Cardiac & Respiratory Issues: Arrhythmia (irregular heartbeat), breathing difficulties, and blood pressure fluctuations.
4. Neurological & Motor Function Loss
- Leg Weakness: Acute loss of motor control or sudden muscle weakness.
- Cognitive Issues: “Brain fog,” speech difficulties, or vision problems.
- Sexual Dysfunction: Caused by nerve damage in the pelvic region.
Manufacturers and Devices Under Investigation
We are reviewing cases involving devices from the major manufacturers dominating the market:
- Abbott (St. Jude Medical): Proclaim, Prodigy, Eterna, BurstDR, Genesis, Eon.
- Note: Abbott recalled 155,000 Proclaim IPGs in 2023 due to issues exiting MRI mode.
- Boston Scientific: Spectra, Precision, Montage, Novi, WaveWriter.
- Medtronic: Intellis, Vanta, Inceptiv.
- Nevro (Globus Medical): Senza, HFX, Omnia.
Do I Qualify for a Spinal Cord Stimulator Lawsuit?
You may be eligible to file a claim if:
- Implantation: You had a Spinal Cord Stimulator implanted.
- Complications: You experienced serious side effects such as burns, shocks, lead migration, infection, bowel/bladder incontinence, or unwanted stimulation.
- Revision Surgery: You underwent (or have been advised to undergo) surgery to remove, replace, or reposition the device.
Note: Studies show that between 40% and 65% of SCS patients require revision surgery within 18 months of implantation. If this happened to you, you have rights.
Potential Compensation in an SCS Lawsuit
Filing a claim is about more than just a settlement; it’s about securing the resources you need for long-term recovery. In 2026, litigation is increasingly focused on “Economic Inflation” in medical care—meaning we fight for awards that account for the rising costs of future surgeries and specialized treatment.
1. Economic Damages (Tangible Losses)
- Medical Expenses: Coverage for past surgeries, hospitalizations, and future “Life Care” costs.
Note: With current inflation trends, the cost to replace an SCS battery or lead can now exceed $25,000–$60,000 per procedure. - Lost Wages & Diminished Earning Capacity: Compensation for the time you’ve missed at work and any permanent inability to return to your previous profession.
- Out-of-Pocket Costs: Transportation to specialists, home health care, and physical therapy.
2. Non-Economic Damages (Quality of Life)
- Pain and Suffering: For the physical agony of internal burns, electrical shocks, or nerve damage.
- Emotional Distress: Compensation for the anxiety, depression, or “medical trauma” caused by a device failing inside your body.
- Loss of Enjoyment of Life: For the hobbies, family activities, and daily routines you can no longer participate in due to injury.
- Permanent Disability: Specific awards if the device caused paralysis, loss of motor control, or permanent organ dysfunction (like bowel/bladder issues).
Why “Future Value” Matters in 2026
In recent filings like Tuttle v. Abbott (Jan 2026), attorneys are putting a massive emphasis on Future Medical Needs. Because these devices are implanted, many victims face a lifetime of “monitoring” or multiple revision surgeries. We ensure your claim accounts for the next 20 to 30 years of care, not just your current bills.
How NGRV Can Help
Nigh Goldenberg Raso & Vaughn is a nationally recognized mass tort law firm with extensive experience holding medical device manufacturers accountable.
When you contact NGRV:
- Your case will be reviewed for free
- There are no upfront costs—you pay nothing unless we win
- Our team handles every step of the legal process
- You get direct access to attorneys experienced in complex medical device litigation
We fight to expose corporate misconduct and pursue justice for injured patients.
Get a Free, Confidential Case Review
The attorneys at Nigh Goldenberg Raso & Vaughn are national leaders in mass tort litigation. We have the resources, experience, and grit to stand up to billion-dollar medical device corporations. We operate on a contingency fee basis, meaning you pay nothing unless we win your case.
Don’t wait—time limits apply to filing your claim.